What’s the “Actionable Gist” of EU GMP for HVAC?
At the heart of all that we usually care about while designing, commissioning, or qualifying HVAC systems for a sterile manufacturing facility are three letters: GMP (Good Manufacturing Practice). From air changes to room pressures to room classification to filtration, all key HVAC requirements ultimately originate from GMP.
GMP comes in many flavors such as FDA, EU, WHO, etc. This post attempts to extract the “actionable gist” of the European Union’s (EU) GMP regulations that relate to HVAC. The words “actionable” and “gist” are important because I don’t intend to regurgitate the regulations, which you can always read anyway. But rather interpret them in terms of specific insights in the context of HVAC to comply with EU GMP.
We will handle this subject in two parts:
- FOREST: Overview of EU GMP regulations. This part gives you a broad picture of the forest of EU regulations.
- TREES: Gist of regulations in relation to HVAC. This is about zeroing in on specific trees that matter from an HVAC point of view.
FOREST: Overview of EU GMP regulations
To get a grip on EU GMP regulations, the elephant to capture is Eudralex, which is one huge collection of all EU rules and regulations covering medicinal products. Eudralex comprises 10 volumes, but for us, the volume to look at is Vol. 4, which is titled “Guidelines for good manufacturing practices for medicinal products for human and veterinary use.”
If we go to Vol. 4, it includes 4 parts and 21 Annexes. Again, we need not get lost in the haystack and must zero in on parts that relate to HVAC:
- Part 1 (Basic Requirements or Medicinal Products) > Chapter 3 – Premises and Equipment under, and
- Annex 1: Manufacture of Sterile Medicinal Products (set to come into force on 25 Aug. 2023)
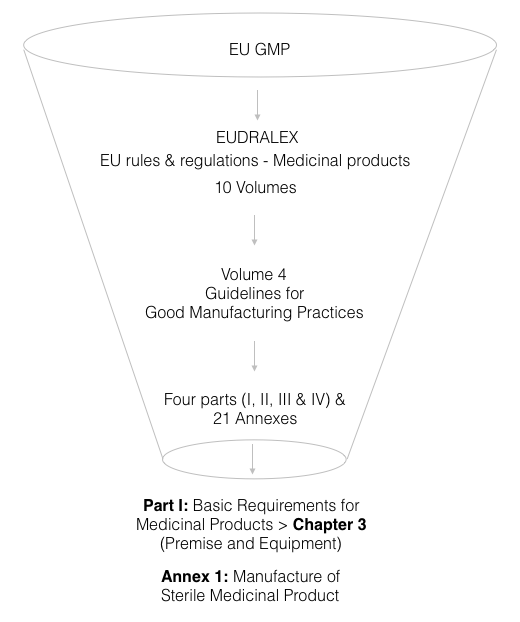
Next, we are going to extract the gist of Chapter 3 of Part 1 and Annex 1 without clouding our minds with the fog of text that these regulations are.
TREES: Gist of Chapter 3 and Annex 1
If you read Chapter 3 of Part I and Annex 1, and ask “What does it all mean from an HVAC point of view,” here is what you’re likely to extract:
Air changes
- Maintain the required number of supply air changes for the Grade A, B, C, and D rooms. [Actual text doesn’t talk about air changes but just says…cleanrooms should be ventilated.]
Supply air filtration
- Provide terminal HEPA filters for Grades A, B, and C to ensure filtered air supply to cleanrooms. For Grade D, provide HEPA inside the Air Handling Unit (AHU). [Actual text doesn’t talk about terminal HEPA but just says…filtered air supply is required and it should be appropriate to the products handled and the operations undertaken.]
Pressure differential
- Adjacent rooms of different grades should have an air pressure difference of a minimum of 10 Pascals. [Actual text does mention the figure of 10 Pa, one of the rare specific pieces of guidance.]
- Pressure differentials (between cleanrooms of different grades) should be (a) displayed, (b) monitored, and (c) recorded.
- A system with appropriate set points, warnings, and alarms should be provided that can instantly warn the operators about the failure of the air supply or reduction of pressure differences.
- Any alarm delays should be assessed and justified.
Airflow pattern visualization
- Make sure supply air outlets and return/exhaust air inlets are well distributed around the cleanroom to provide a good sweeping type airflow pattern.
- Provide low-wall return/exhaust air inputs in grade A, B, and C areas.
- Airflow patterns should be visualized with video recording for:
- Airflow between rooms of different grades (to demonstrate that there is no ingress from lower grade to higher grade areas).
- Within the cleanroom to show that air doesn’t travel from less clean areas (floor, operator, equipment) to transfer contamination to cleaner process/working areas.
- Unidirectional Airflow areas.
[The first two points above are my interpretation. The actual text only talks about the last bullet point…airflow pattern visualization.]
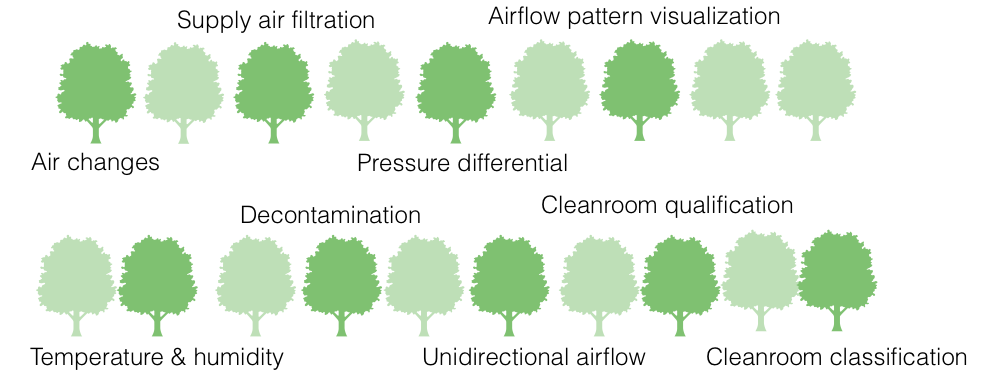
Temperature and humidity
- Temperature and humidity should be maintained to suit the product and operations.
Decontamination
- Provide decontamination of HVAC systems and the treatment of air leaving a clean area where needed.
Unidirectional airflow velocity
- Unidirectional airflow systems should provide a homogeneous air speed in a range of 0.36 – 0.54 m/s (guidance value) at the working position.
Tests for cleanroom qualification
- Installed filter system leakage and integrity testing
- Airflow tests – volume and velocity
- Air pressure difference test
- Airflow direction test and visualization
- Microbial airborne and surface contamination
- Temperature measurement test
- Relative humidity test
- Recovery test
- Containment leak test
Cleanroom classification
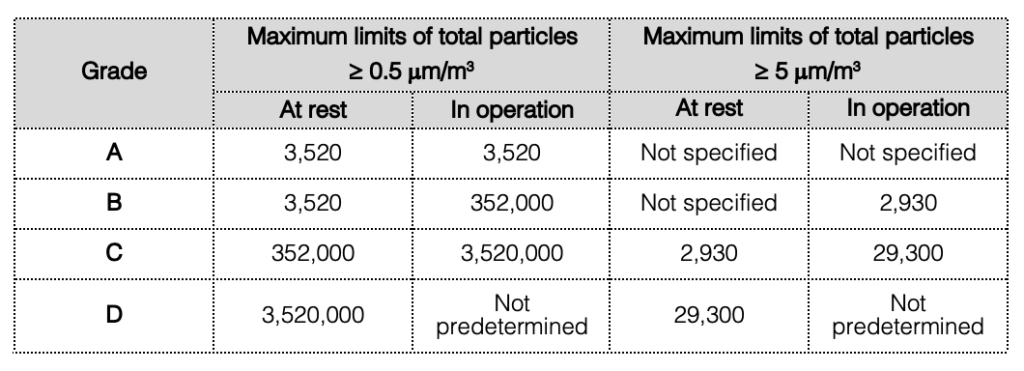
For cleanroom classification, the total of particles equal to or greater than 0.5 μm and 5 μm should be measured. This measurement should be performed both at rest and in simulated operations in accordance with the limits specified in the table below.
To conclude, I don’t see anything special about these guidelines. More or less, every project team follows them whether they have read EU GMP or not except for three items:
- Taking good care of airflow patterns at the design stage. Due to design constraints or sheer ignorance, this item tends to get compromised, which is risky.
- Visualizing airflow patterns during qualification. Some people don’t know about it or think it is optional, but that’s not the case.
- Including a GMP alarm for the loss of supply airflow. Again, I find this is missing in some projects.
Finally, leaving you with a point to ponder: EU guidelines require air speed in a range of 0.36 – 0.54 m/s at the working position. Is that practical?
In case you’re keen to dive into the forest of EU GMP, here are the links:
- EudraLex – Volume 4 – Good Manufacturing Practice (GMP) guidelines
- Annex 1: Manufacture of Sterile Medicinal Products
