HVAC for Biopharma Professionals
2-Day Comprehensive Training (Online)
17 Nov & 20 Nov 2023
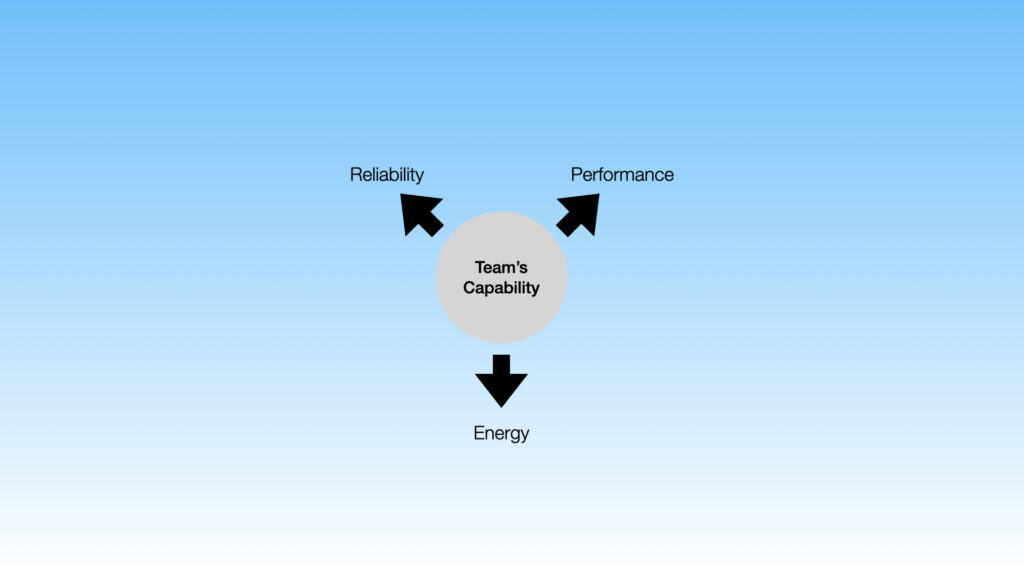
HVAC systems maintain critical GMP environmental conditions (temperature, humidity, cleanliness, pressures) in biopharma facilities. The performance and reliability of these systems are essential to site operations. Consuming 50-60% of overall energy, these systems also have a direct impact on the cost of operations and sustainability.
In the end, however, all three objectives (performance, reliability, and energy) depend on a crucial ingredient: The capability of the team that operates and manages the HVAC systems.
This training has been specially designed to enhance that capability.
Target Audience
- Senior engineers, engineers, supervisors, senior technicians
- Involved in HVAC maintenance & operations, projects, commissioning & qualification
Training Objectives
- Fundamentals: Consolidate HVAC fundamentals relevant to routine operations
- Pharma HVAC & GMP: Gain understanding of HVAC design configurations for biopharma plants and applicable GMP regulations and standards
- Troubleshooting: Learn how to systematically troubleshoot HVAC problems
- Energy: Identify various energy-saving opportunities in pharma HVAC systems
Training outline
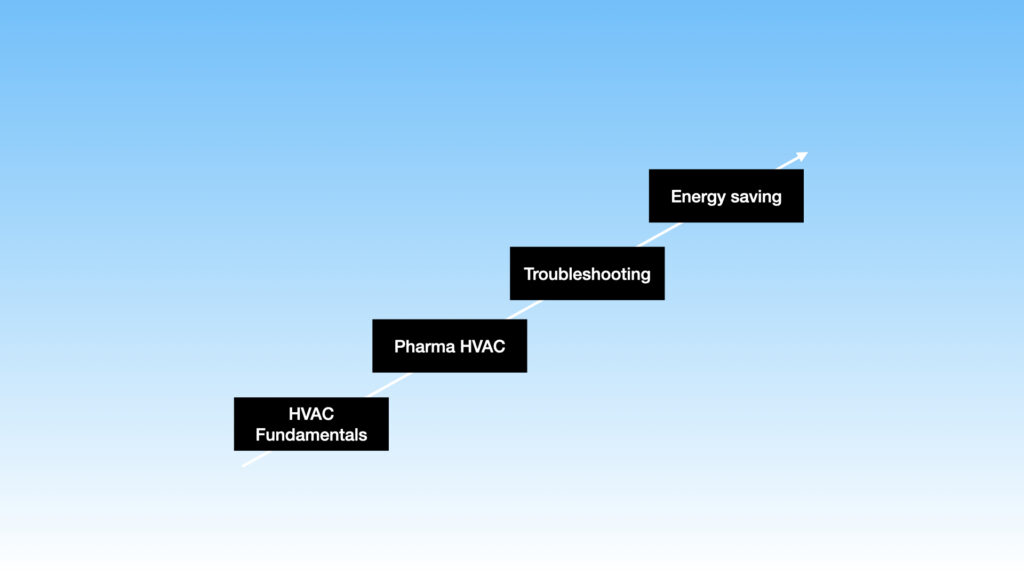
The training focuses on the following four key areas:
- Fundamentals
- Pharma HVAC
- Troubleshooting
- Energy saving
The details are as follows:
Module 1 (Fundamentals): Key HVAC Principles
- Application of HVAC fundamentals for operating a plant
- Vapor compression cycle
- P-H diagram, COP, and concept of “Lift”
- Fan curves & fan laws
- Pump curves & pump laws
Module 2: Psychrometry
- Properties of air (Dry bulb and wet bulb temperatures, relative humidity, enthalpy, etc.)
- Reading & using a Psychrometric Chart
- Psychrometric calculations
Module 3: Pharma HVAC
- Important user requirements
- Pharma design considerations and configurations for different applications
- Reviewing HVAC design, installation & commissioning as end-users
- Latest trends (including AI)
Module 4: GMP Regulations & Standards
- ISO and ISPE guidelines
- EU GMP, WHO, FDA requirements
- ASHRAE and local codes
Module 5: HVAC Troubleshooting Strategies
- Typical HVAC problems in biopharma plant
- Two types of problems – known and unknown causes
- Why jumping to solutions is easy but costly
- Systematic approach
- Examine
- Analyze (Five Why’s, Fishbone)
- Solve
Module 6: Troubleshooting with a Checklist
- Power of a checklist (WHO example)
- How to create a powerful checklist for troubleshooting
- Collaborative troubleshooting
- Preventing problems
- Case studies
Module 7: Energy Saving (Part 1)
- Drivers for saving energy
- Key terms: COP, kW/TR, EER & basic energy calculations
- ISO 14644-16 (energy efficiency in cleanrooms)
- Key HVAC-related strategies (cleanroom side)
- Air changes (ACH) reduction
- Temperature and humidity set points
- Night/weekend setback
- Cleanroom tightness
Module 8: Energy Saving (Part 2)
- Key HVAC-related strategies (plant side)
- Fresh airflow reduction
- High-efficiency equipment: Fans, motors
- Tightness – ducts
- Design parameters: Outdoor conditions, equipment selection, filter selection
- VFD
- Heat recovery, run around coil
- How
- Energy management – a systematic approach, stakeholders, conflicting
requirements (GMP, reliability vs. cost savings), risk-based approach
- Energy management – a systematic approach, stakeholders, conflicting
- Case study
Training Schedule
- 17 Nov 2023 (Friday): 8:30 am – 5:00 pm (Singapore time)
- 20 Nov 2023 (Monday): 8:30 am – 5:00 pm (Singapore time)
Trainer: Atul Mathur
- Overall 30 years of experience in HVAC and the last 15 years in pharma HVAC (design, commissioning, qualification, and project management)
- ACTA-certified trainer with 15 years of training experience
- Master’s degree in engineering
- For more information, please visit: Atul Mathur’s LinkedIn profile page
Features
- Relevant & Practical: Designed & delivered by a practitioner with decades of experience for other fellow practitioners
- Interactive: Highly engaging with questions, discussions, exercises, and experience-sharing…it’s not a boring slide show
- Competency-based: Every module starts with clear learning goals and ends with a small quiz…you’ll need to be alert and engaged
- High-quality: Precise and high-quality content and training materials
Benefits
By attending this 2-day training, you’ll learn:
- HVAC fundamental concepts useful for routine operations;
- Different types of pharma HVAC designs and GMP regulations and international standards;
- Systematic approach to troubleshooting; and
- energy saving opportunities in pharma HVAC systems.
Bottomline: Back from the training, you’ll do your job with greater confidence and competence.
Fee
- Standard fee: SGD 880/pax
- Discounted fee – early registration by 10 Nov: SGD 680/pax
- Discounted fee – Group (min. 2 pax): SGD 480/pax
How to register (1-2-3)
- To register, simply send an email to: atul@atulmathur.com with the following details
- (i) Full Name, (ii) designation, (iii) company name, and (iv) number of participants (in case of group registration)
- You’ll receive an acknowledgment with payment details
- Make the payment to confirm your registration
View and download the above content in slides…
