What Will You Get if You Pass FDA’s GMP Regulations Through a Special HVAC Filter
If you enter a forest but do not know what to look for or where to go, chances are you’ll either get lost or simply have a random trip. GMP regulations by the FDA, EU, WHO, etc. are a couple of such huge forests. Comprising hundreds of documents and thousands of clauses, it’s an interesting challenge to navigate and not get lost in the fog of dense text.
This post takes you on a hike through the FDA’s GMP forest with the sole intention of coming back with some beautiful leaves, flowers, and fruits that look like HVAC-related GMP.
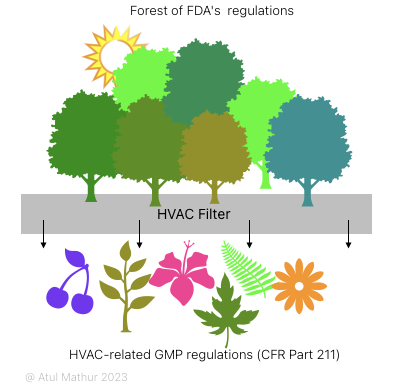
We will handle this subject in two parts:
- FOREST: Overview of FDA regulations. This part gives you a broad picture of the forest of FDA regulations and the path to GMP regulations.
- TREES: Gist of GMP regulations in relation to HVAC. This is zeroing in on specific items that matter from an HVAC point of view.
FOREST: Overview of FDA’s regulations
To navigate to FDA’s GMP regulations, the starting point is a huge tree with thousands of branches. Known as CFR (Code of Federal Regulations), it is a collection of “Titles” from 1 to 50. But for us, Title 21 (Food and Drugs) matters. Within Title 21, there are three chapters, of which Chapter 1 is of relevance. If you dig deeper, you will realize although Chapter 1 comprises subchapters from A to L, what matters is Subchapter C: Drugs – General. Like a set of Russian dolls, if take a close look at Subchapter C, you will find it includes 100 parts (numbered from 200 to 299) of which the part of our interest is…just this:
- Part 211: Current Good Manufacturing Practice for Finished Pharmaceuticals
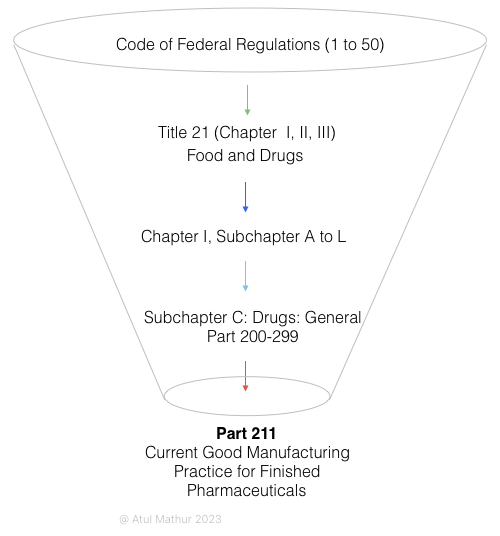
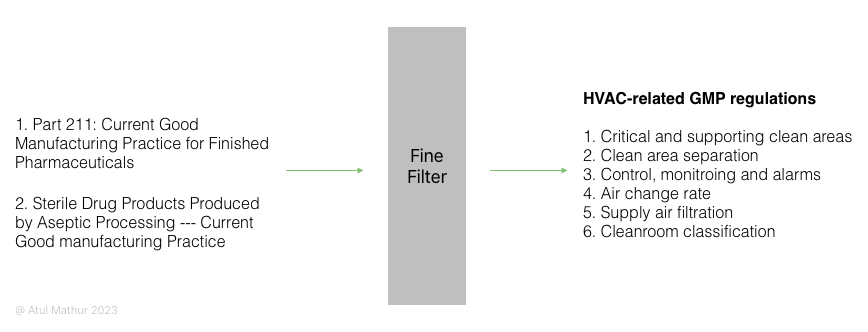
Considering CFR is too high-level, the FDA has another more detailed guidance document titled “Guidance for Industry: Sterile Drug Products Produced by Aseptic Processing – Current Good Manufacturing Practice.”
So we have two relevant documents (Part 211 and the guidance document). What do we get if comb through these from an HVAC point of view?
TREES: Gist of Part 211 & FDA cGMP guidance document
If you pass Part 211 (Subpart C – Buildings and Facilities) and GMP guidance document through an HVAC filter, you’ll get six HVAC-related items on the other side:
- Critical and supporting clean areas
- Clean area separation
- Control, monitoring, and alarms
- Air change rate
- Supply air filtration
- Cleanroom classification
So, let’s come to the bottom line: What’s inside those six points?
1. Critical and supporting clean areas
- Two clean areas are of particular importance to sterile drug product quality: the critical area and the supporting clean areas associated with it.
- Critical area
- A critical area is one in which the sterilized drug product, containers, and closures are exposed to environmental conditions that must be designed to maintain product sterility. Activities conducted in such areas include manipulations (e.g., aseptic connections, sterile ingredient additions) of sterile materials prior to and during filling and closing operations.
- Cleanliness classification for critical areas: Class 100 (ISO 5).
- HEPA-filtered air should be supplied in critical areas at a velocity sufficient to sweep particles away from the filling/closing area and maintain unidirectional airflow during operations.
- In situ air pattern analysis should be conducted at the critical area to demonstrate unidirectional airflow and sweeping action over and away from the product under dynamic conditions.
- Supporting clean areas
- Many support areas function as zones in which nonsterile components, formulated products, in-process materials, equipment, and container/closures are prepared, held, or transferred.
- The nature of the activities conducted in a supporting clean area determines its classification.
- FDA recommends that the area immediately adjacent to the aseptic processing line meet, at a minimum, Class 10,000 (ISO 7) standards under dynamic conditions. Manufacturers can also classify this area as Class 1,000 (ISO 6) or maintain the entire aseptic filling room at Class 100 (ISO 5).
- An area classified at a Class 100,000 (ISO 8) air cleanliness level is appropriate for less critical activities (e.g., equipment cleaning).
2. Clean area separation
- A positive pressure differential of at least 10-15 Pascals (Pa) should be maintained between adjacent rooms of differing classification (with doors closed). When doors are open, outward airflow should be sufficient to minimize ingress of contamination, and it is critical that the time a door can remain ajar be strictly controlled.
- In some cases, the aseptic processing room and adjacent cleanrooms have the same classification. Maintaining a pressure differential (with doors closed) between the aseptic processing room and these adjacent rooms can provide beneficial separation.
- In any facility designed with an unclassified room adjacent to the aseptic processing room, a substantial overpressure (e.g., at least 12.5 Pa) from the aseptic processing room should be maintained at all times to prevent contamination.
3. Control, monitoring, and alarms
- The pressure differentials between cleanrooms should be monitored continuously throughout each shift and frequently recorded.
- All alarms should be documented and deviations from established limits should be investigated.
- Temperature and humidity controls should provided.
- A system for monitoring environmental conditions should be provided.
4. Air change rate
- For Class 100,000 (ISO 8) supporting rooms, airflow sufficient to achieve at least 20 air changes per hour is typically acceptable. Significantly higher air change rates are normally needed for Class 10,000 and Class 100 areas.
5. Supply air filtration
- HEPA filter integrity should be maintained to ensure aseptic conditions. Leak testing should be performed at installation to detect integrity breaches around the sealing gaskets, through the frames, or through various points on the filter media.
- HEPA filter leak testing alone is insufficient to monitor filter performance. It is important to conduct periodic monitoring of filter attributes such as uniformity of velocity across the filter (and relative to adjacent filters).
- If air is recirculated to production areas, measures shall be taken to control recirculation of dust from production.
- In areas where air contamination occurs during production, there shall be adequate exhaust systems or other systems adequate to control contaminants.
6. Cleanroom classification
The table below summarizes the clean air classifications.
Air Classifications
| Clean Area Classification (0.5 µm particles/ft3) | ISO Designation | ≥ 0.5 µm particles/m2 (In Operation) |
| 100 | 5 | 3,520 |
| 1000 | 6 | 35,200 |
| 10,000 | 7 | 352,000 |
| 100,000 | 8 | 3,520,000 |
To conclude, you’ll realize FDA’s GMP regulations look at things from 40,000 feet. I am sure most people in the industry know these basics whether they have ever been on a trip to the FDA GMP forest or not.
Finally, you can also see how effective are HVAC filters! It can filter the whole FDA GMP down to six simple points.
Simple can be harder than complex: You have to work hard to get your thinking clean to make it simple. But it’s worth it in the end because once you get there, you can move mountains.
Steve Jobs
FDA GMP links:
- Part 211: Current Good Manufacturing Practice for Finished Pharmaceuticals
- Sterile Drug Products Produced by Aseptic Processing – Current Good Manufacturing Practice
Upcoming…
I will be offering a training program titled “HVAC for Biopharma Professionals” next month. Check it out here.
